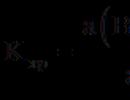Lesson "soluble and insoluble substances in water." What dissolves in water? What substances are insoluble in water
Solution is a thermodynamically stable homogeneous (single-phase) system of variable composition, consisting of two or more components (chemicals). The components that make up a solution are a solvent and a solute. Typically, a solvent is considered to be a component that, in its pure form, exists in the same state of aggregation as the resulting solution (for example, in the case of an aqueous solution of salt, the solvent is, of course, water). If both components were in the same state of aggregation before dissolution (for example, alcohol and water), then the component that is in larger quantity is considered the solvent.
Solutions are liquid, solid and gaseous.
Liquid solutions are solutions of salts, sugar, alcohol in water. Liquid solutions can be aqueous or non-aqueous. Aqueous solutions are solutions in which the solvent is water. Non-aqueous solutions are solutions in which the solvents are organic liquids (benzene, alcohol, ether, etc.). Solid solutions are metal alloys. Gaseous solutions - air and other mixtures of gases.
Dissolution process. Dissolution is a complex physical and chemical process. During the physical process, the structure of the solute is destroyed and its particles are distributed between the solvent molecules. A chemical process is the interaction of solvent molecules with solute particles. As a result of this interaction, solvates. If the solvent is water, the resulting solvates are called hydrates. The process of formation of solvates is called solvation, the process of formation of hydrates is called hydration. When aqueous solutions are evaporated, crystalline hydrates are formed - these are crystalline substances that contain a certain number of water molecules (water of crystallization). Examples of crystalline hydrates: CuSO 4 . 5H 2 O – copper (II) sulfate pentahydrate; FeSO4 . 7H 2 O – iron (II) sulfate heptahydrate.
The physical process of dissolution occurs with absorption energy, chemical - with highlighting. If, as a result of hydration (solvation), more energy is released than is absorbed during the destruction of the structure of a substance, then dissolution is exothermic process. Energy is released when NaOH, H 2 SO 4, Na 2 CO 3, ZnSO 4 and other substances are dissolved. If more energy is needed to destroy the structure of a substance than is released during hydration, then dissolution is endothermic process. Energy absorption occurs when NaNO 3, KCl, NH 4 NO 3, K 2 SO 4, NH 4 Cl and some other substances are dissolved in water.
The amount of energy that is released or absorbed during dissolution is called thermal effect of dissolution.
Solubility a substance is its ability to be distributed in another substance in the form of atoms, ions or molecules to form a thermodynamically stable system of variable composition. A quantitative characteristic of solubility is solubility coefficient, which shows what maximum mass of a substance can dissolve in 1000 or 100 g of water at a given temperature. The solubility of a substance depends on the nature of the solvent and substance, on temperature and pressure (for gases). The solubility of solids generally increases with increasing temperature. The solubility of gases decreases with increasing temperature, but increases with increasing pressure.
Based on their solubility in water, substances are divided into three groups:
1. Well soluble (r.). The solubility of substances is more than 10 g in 1000 g of water. For example, 2000 g of sugar dissolves in 1000 g of water, or in 1 liter of water.
2. Slightly soluble (m.). The solubility of substances is from 0.01 g to 10 g in 1000 g of water. For example, 2 g of gypsum (CaSO 4 . 2 H 2 O) dissolves in 1000 g of water.
3. Practically insoluble (n.). The solubility of substances is less than 0.01 g in 1000 g of water. For example, 1.5 dissolves in 1000 g of water . 10 -3 g AgCl.
When substances dissolve, saturated, unsaturated and supersaturated solutions can form.
Saturated solution is a solution that contains the maximum amount of solute under given conditions. When a substance is added to such a solution, the substance no longer dissolves.
Unsaturated solution- a solution that contains less solute than a saturated solution under given conditions. When a substance is added to such a solution, the substance still dissolves.
Sometimes it is possible to obtain a solution that contains more solute than a saturated solution at a given temperature. Such a solution is called supersaturated. This solution is prepared by carefully cooling the saturated solution to room temperature. Supersaturated solutions are very unstable. Crystallization of a substance in such a solution can be caused by rubbing the walls of the vessel in which the solution is located with a glass rod. This method is used when performing some qualitative reactions.
The solubility of a substance can also be expressed by the molar concentration of its saturated solution (section 2.2).
Solubility constant. Let us consider the processes that arise during the interaction of the poorly soluble but strong electrolyte of barium sulfate BaSO 4 with water. Under the influence of water dipoles, Ba 2+ and SO 4 2 - ions from the BaSO 4 crystal lattice will pass into the liquid phase. Simultaneously with this process, under the influence of the electrostatic field of the crystal lattice, some of the Ba 2+ and SO 4 2 - ions will be deposited again (Fig. 3). At a given temperature, equilibrium will finally be established in the heterogeneous system: the rate of the dissolution process (V 1) will be equal to the rate of the precipitation process (V 2), i.e.
BaSO 4 ⇄ Ba 2+ + SO 4 2 -
solid solution
Rice. 3. Saturated barium sulfate solution
A solution in equilibrium with the solid phase BaSO 4 is called rich relative to barium sulfate.
A saturated solution is an equilibrium heterogeneous system, which is characterized by a chemical equilibrium constant:
 , (1)
, (1)
where a (Ba 2+) is the activity of barium ions; a(SO 4 2-) – activity of sulfate ions;
a (BaSO 4) – activity of barium sulfate molecules.
The denominator of this fraction - the activity of crystalline BaSO 4 - is a constant value equal to unity. The product of two constants gives a new constant called thermodynamic solubility constant and denote K s °:
К s° = a(Ba 2+) . a(SO 4 2-). (2)
This quantity was previously called the solubility product and designated PR.
Thus, in a saturated solution of a sparingly soluble strong electrolyte, the product of the equilibrium activities of its ions is a constant value at a given temperature.
If we assume that in a saturated solution of a sparingly soluble electrolyte the activity coefficient f~1, then the activity of ions in this case can be replaced by their concentrations, since a( X) = f (X) . WITH( X). The thermodynamic solubility constant K s ° will turn into the concentration solubility constant K s:
K s = C(Ba 2+) . C(SO 4 2-), (3)
where C(Ba 2+) and C(SO 4 2 -) are the equilibrium concentrations of Ba 2+ and SO 4 2 - ions (mol/l) in a saturated solution of barium sulfate.
To simplify calculations, the concentration solubility constant K s is usually used, taking f(X) = 1 (Appendix 2).
If a poorly soluble strong electrolyte forms several ions upon dissociation, then the expression K s (or K s °) includes the corresponding powers equal to the stoichiometric coefficients:
PbCl 2 ⇄ Pb 2+ + 2 Cl - ; K s = C (Pb 2+) . C 2 (Cl -);
Ag 3 PO 4 ⇄ 3 Ag + + PO 4 3 - ; K s = C 3 (Ag +) . C (PO 4 3 -).
In general, the expression for the concentration solubility constant for an electrolyte is A m B n ⇄ m A n+ + n B m - has the form
K s = С m (A n+) . C n (B m -),
where C is the concentration of A n+ and B m ions in a saturated electrolyte solution in mol/l.
The K s value is usually used only for electrolytes whose solubility in water does not exceed 0.01 mol/l.
Conditions for precipitation formation
Let us assume that c is the actual concentration of ions of a sparingly soluble electrolyte in the solution.
If C m (A n +) . With n (B m -) > K s, then the formation of a precipitate will occur, because the solution becomes supersaturated.
If C m (A n +) . C n (B m -)< K s , то раствор является ненасыщенным и осадок не образуется.
Properties of solutions. Below we will consider the properties of non-electrolyte solutions. In the case of electrolytes, an isotonic correction factor is introduced into the given formulas.
If a non-volatile substance is dissolved in a liquid, then the saturated vapor pressure above the solution is less than the saturated vapor pressure above the pure solvent. Simultaneously with a decrease in the vapor pressure above the solution, a change in its boiling and freezing points is observed; The boiling points of solutions increase, and the freezing temperatures decrease compared to the temperatures characterizing pure solvents.
The relative decrease in the freezing point or the relative increase in the boiling point of a solution is proportional to its concentration.
You can do the following experiments with water at home:
Pour a teaspoon of granulated sugar into a glass of water and stir it. What happens to grains of sand? Where did they go? Can we say that granulated sugar has disappeared (taste the water). Has the color of the water in which you stirred the sand changed? Has it lost its transparency?
Strain the sweet water through a paper filter. Taste it. Has the water been cleared of the sugar mixed in it?
Pour a teaspoon of cleanly washed river sand into a glass of water and stir it. Does anything happen to grains of sand in water? Has the color and clarity of the water changed?
Strain the water with river sand through a paper filter. Is river sand removed from water using a filter?
There is such a fairy tale. Two donkeys were walking along the road with luggage. One was loaded with salt, and the other with cotton wool. The first donkey could hardly move his legs: his burden was so heavy. The second one was fun and easy.
Soon the animals had to cross the river. The donkey, loaded with salt, stopped in the water and began to swim: he first lay down in the water, then stood on his feet again. When the donkey came out of the water, his burden became much lighter. The other donkey, looking at the first one, also began to bathe. But the longer he bathed, the heavier the cotton wool loaded onto him became.
Why did the burden of the first donkey become lighter after bathing, and heavier for the second? What would happen if the second donkey carried sugar instead of cotton wool?
Experiments will help you answer these questions:
Pour pure salt into a glass of water and stir it with a spoon. Watch what happens to the salt crystals. They become smaller and smaller and soon disappear completely. But has the salt disappeared? Taste the water. It's salty. The salt did not disappear, but became invisible. She disappeared.
Pass the water through the filter. Nothing settles on the filter, and the water remains salty.
Remember the experiment with sugar that you did before reading the article. When you stir sugar in water, it also becomes invisible, that is, it dissolves.
Do the same experiment with baking soda as you did with sugar and salt. Does soda dissolve in water?
When you performed an experiment with river sand at home, you observed that the grains of sand fell to the bottom of the glass and lay there unchanged. You ran water through a filter. The water passed through it, but the sand remained on the filter. From this experiment we can conclude that sand does not dissolve in water.
Try dissolving clay and tooth powder. Particles of these substances will float in the water, which becomes cloudy from them. If you let the water sit, particles of clay and tooth powder will settle to the bottom. When you stir the water, they will rise and then fall again.
Pass the cloudy water through a paper filter. The water will become clean and clear, and particles of clay and tooth powder will remain on the filter. This means that these substances, like sand, do not dissolve in water.
Now you can take any substance yourself and check whether it dissolves or not. If its particles become invisible in the water and pass through the filter with it, then it is a soluble substance.
If particles float in the water or settle to the bottom and are retained by the filter, then it is an insoluble substance. Water in which any substance is dissolved is called a solution.
Useful on the web
A manual coffee grinder will help you prepare aromatic, delicious coffee. Why is the coffee grinder manual, you ask. The fact is that only when using a manual coffee grinder do the beans release the full range of flavor into the drink. Coffee grinders have a grinding degree regulator, and they are made of glass and wood.
In an ordinary non-associated liquid, such as gasoline, the molecules slide freely around one another. In water, they roll rather than glide. Water molecules are known to be connected to each other by hydrogen bonds, so before any displacement occurs, at least one of these bonds must be broken. This feature determines the viscosity of water.
The dielectric constant of water is its ability to neutralize the attraction that exists between electric charges. The dissolution of solids in water is a complex process that is determined by the interaction of solute particles and water particles.
When studying the structure of substances using X-rays, it was found that most solids have a crystalline structure, that is, the particles of the substance are located in space in a certain order. Particles of some substances are located as if they were in the corners of a tiny cube, particles of others - in the corners, center and middle of the sides of a tetrahedron, prism, pyramid, etc. Each of these forms is the smallest cell of larger crystals of a similar shape. Some substances have molecules at the nodes of their crystal lattice (most organic compounds), others (for example, inorganic salts) have ions, i.e. particles consisting of one or more atoms with positive or negative charges. The forces that hold ions in a certain spatially oriented order of the crystal lattice are the forces of electrostatic attraction of oppositely charged ions that make up the crystal lattice.
If, for example, sodium chloride is dissolved in water, then the positively charged sodium ions and negatively charged chlorine ions will repel each other.
This repulsion occurs because water has a high dielectric constant, i.e. higher than that of any other liquid. It reduces the force of mutual attraction between oppositely charged ions by 100 times. The reason for the strong neutralizing effect of water must be sought in the arrangement of its molecules. The hydrogen atom in them does not share its electron equally with the oxygen atom to which it is attached. This electron is always closer to oxygen than to hydrogen. Therefore, hydrogen atoms are positively charged, and oxygen atoms are negatively charged.
When a substance dissolves into ions, oxygen atoms are attracted to positive ions, and hydrogen atoms are attracted to negative ions. The water molecules surrounding the positive ion send their oxygen atoms towards it, and the molecules that surround the negative ion send their hydrogen atoms towards it. Thus, water molecules form a kind of lattice, which separates the ions from each other and neutralizes their attraction (Fig. 12). In order to separate the ions located in the crystal lattice from each other and transfer them into solution, it is necessary to overcome the attractive force of this lattice. When dissolving salts, this force is the attraction of lattice ions by water molecules, characterized by the so-called hydration energy. If the hydration energy is sufficiently high compared to the energy of the crystal lattice, then the ions will break away from the latter and go into solution.
The relationship between water molecules and ions separated from the lattice in solution not only does not weaken, but becomes even closer.
As already noted, in a solution, ions are surrounded and separated by water molecules, which, focusing on them with their parts opposite in charge, form the so-called hydration shell (Fig. 13). The size of this shell is different for different ions and depends on the charge of the ion, its size and, in addition, on the concentration of ions in the solution.
For several years, physical chemists studied water mainly as a solvent for electrolytes. As a result, much information has been obtained about electrolytes, but very little about water itself. Oddly enough, only in recent years have works appeared devoted to the study of the relationship of water to substances that are practically insoluble in it.
Many amazing phenomena were observed. For example, one day a pipe carrying natural gas at t = 19°C turned out to be clogged with wet snow and water. It became clear that the problem here was not the temperature, but other properties of water. A number of questions arose: why water froze at such a high temperature, how water could combine with substances that are insoluble in it.
This mystery had not yet been solved when it was discovered that even such noble gases as argon and xenon, which do not enter into any chemical reactions, can combine with water, forming some semblance of compounds.

Rice. 13. Separation of Na + and C1 - ions by polar water molecules, forming a hydration shell around them.
Interesting results on the solubility of methane in water were obtained in Illinois. Methane molecules do not form ions in water and do not perceive hydrogen bonds; the attraction between them and water molecules is very weak. However, methane still, although poorly, dissolves in water, and its dissociated molecules form compounds with it - hydrates, in which several water molecules are attached to one methane molecule. This reaction releases 10 times more heat than dissolving methane in hexane (methane dissolves better in hexane than in water).
The fact that methane dissolves in water is of great interest. After all, a methane molecule has twice the volume of a water molecule. For methane to dissolve in water, quite large “holes” must form between its molecules. This requires a significant expenditure of energy, greater than for the evaporation of water (approximately 10,000 calories for each mole). Where does so much energy come from? The attractive forces between methane and water molecules are too weak, they cannot provide as much energy. Therefore, another possibility exists: the structure of the hearth changes in the presence of methane. Let's assume that a molecule of dissolved methane is surrounded by a shell of 10-20 water molecules. When such molecular associations form, heat is released. In the space occupied by a methane molecule, the forces of mutual attraction between water molecules, and hence the internal pressure, disappear. In such conditions, as we have seen, water freezes at temperatures above zero.
This is why molecules in the gap between methane and water can crystallize, which is what happened in the case described above. Frozen hydrates can be absorbed into the solution and released from it. This theory is known as the iceberg theory. In practice, as studies show, all non-conducting substances that were tested form stable crystalline hydrates. At the same time, this tendency is weakly expressed in electrolytes. All this leads to a completely new understanding of solubility.
It was believed that the dissolution of electrolytes occurs as a result of attractive forces. Now it has been proven that the dissolution of non-electrolytes occurs not due to the forces of attraction between these substances and water, but as a result of insufficient attraction between them. Substances that do not break down into ions combine with water, as they eliminate internal pressure and thereby contribute to the appearance of crystalline formations.
To better understand the formation of such hydrates, it is useful to consider their molecular structure.
It has been proven that the resulting hydrates have a cubic structure (lattice) in contrast to the hexagonal structure of ice. Further work by researchers showed that the hydrate can have two cubic lattices: in one of them the gaps between molecules are 12, in the other - 17 A. In the smaller lattice there are 46 water molecules, in the larger one 136. The holes of gas molecules in the smaller lattice have 12-14 faces , and in larger ones - 12-16, moreover, they vary in size and are filled with molecules of different sizes, and not all holes may be filled. This model explains the actual structure of hydrates with a high degree of accuracy.
The role of such hydrates in life processes can hardly be overestimated. These processes occur mainly in the spaces between water and protein molecules. Water has a strong tendency to crystallize, since the protein molecule contains many non-ionic, or non-polar, groups. Any such hydrate forms at a lower density than ice, so its formation can lead to significant destructive expansion.
So, water is a unique and complex substance with certain and varied chemical properties. She has a slender and at the same time changing physical structure.
The development of all living and, to a large extent, inanimate nature is inextricably linked with the characteristic features of water.
Task: Show children the solubility and insolubility of various substances in water.
Materials: flour, granulated sugar, river sand, food coloring, washing powder, glasses of clean water, spoons or chopsticks, trays, pictures depicting the substances presented.
Description. In front of the children on trays are glasses of water, chopsticks, spoons and substances in various containers. Children look at water and remember its properties. What do you think will happen if granulated sugar is added to water? Grandfather Know adds sugar, mixes, and everyone observes together what has changed. What happens if we add river sand to the water? Adds river sand to the water and mixes. Has the water changed? Did it become cloudy or remain clear? Has the river sand dissolved?
What will happen to water if we add food coloring to it? Adds paint and mixes. What changed? (The water has changed color.) Has the paint dissolved? (The paint dissolved and changed the color of the water, the water became opaque.)
Will flour dissolve in water? Children add flour to the water and mix. What did the water become? Cloudy or clear? Has the flour dissolved in the water?
Will washing powder dissolve in water? Add washing powder and mix. Did the powder dissolve in water? What did you notice that was unusual? Dip your fingers into the mixture and check if it still feels the same as clean water? (The water has become soapy.) What substances have dissolved in our water? What substances do not dissolve in water?
(The results are recorded on a flannelgraph.)
COLORED SAND
Tasks: introduce children to the method of making colored sand (mixing with colored chalk); teach how to use a grater.
Materials: colored crayons, sand, transparent container, small objects, 2 bags, small graters, bowls, spoons (sticks), small jars with lids.
Description. The little jackdaw, Curiosity, flew to the children. He asks the children to guess what is in his bags. Children try to determine by touch. (In one bag there is sand, in the other there are pieces of chalk.) The teacher opens the bags, the children check their guesses. The teacher and the children examine the contents of the bags. What is this? What sand? What can you do with it? What color is chalk? What does it feel like? Can it be broken? What is it for? Little Gal asks: “Can sand be colored? How to make it colored? What happens if we mix sand with chalk? How can you make chalk as free-flowing as sand?” Little Gal boasts that he has a tool for turning chalk into fine powder.
Shows the children a grater. What is this? How to use it? Children, following the example of the little jackdaw, take bowls, graters and rub chalk. What happened? What color is your powder? (The little pebble asks each child) How can I make the sand colored now? Children pour sand into a bowl and mix it with spoons or chopsticks. Children look at colored sand. How can we use this sand? (Make beautiful pictures.)
Little Galchoff offers to play. Shows a transparent container filled with multi-colored layers of sand and asks the children: “How can you quickly find a hidden object?” Children offer their own options. The teacher explains that you cannot mix the sand with your hands, a stick or a spoon, and shows how to push an object out of the sand by shaking the vessel.
What happened to the colorful sand? The children note that in this way we quickly found the object and mixed the sand.
Children hide small objects in transparent jars, cover them with layers of multi-colored sand, close the jars with lids and show the little girl how they quickly find the hidden object and mix the sand. Little Galchon gives the children a box of colored chalk as a farewell gift.
GAMES WITH SAND
Tasks: consolidate children’s ideas about the properties of sand, develop curiosity and observation, activate children’s speech, and develop constructive skills.
Materials: a large children's sandbox, in which traces of plastic animals are left, animal toys, scoops, children's rakes, watering cans, a plan of the area for walks of this group.
Description. Children go outside and explore the walking area. The teacher draws their attention to unusual footprints in the sandbox. Why are footprints so clearly visible in the sand? Whose tracks are these? Why do you think so?
Children find plastic animals and test their guesses: they take toys, place their paws on the sand and look for the same print. What trace will be left from the palm? Children leave their marks. Whose palm is bigger? Whose is smaller? Check by applying.
The teacher finds a letter in the bear cub's paws and takes out a site plan from it. What is shown? Which place is circled in red? (Sandbox.) What else could be interesting there? Perhaps some kind of surprise? Children, plunging their hands into the sand, look for toys. Who is this?
Each animal has its own home. The fox has... (hole), the bear has... (den), the dog has... (kennel). Let's build a sand house for each animal. What sand is best for building with? How to make it wet?
Children take watering cans and water the sand. Where does the water go? Why did the sand become wet? Children build houses and play with animals.
Water is a solvent
a liquid substance in which other substances are dissolved a substance that has dissolved in a solvent Solute Solvent Excellent solvent
We want to find out. Many substances in water can disintegrate into invisible tiny particles, that is, dissolve. Therefore, water is a good solvent for many substances. I propose to conduct experiments and identify methods by which it will be possible to obtain an answer to the question of whether a substance dissolves in water or not. What are we taking? What are we seeing? Salt? Granulated sugar? River sand? Clay? What does solubility depend on (experiment)?
Solubility is the content of solute in a saturated solution. There are:
Let's conduct an experiment: Fill a transparent glass with boiled water. Pour a teaspoon of table salt into it. While stirring the water, observe what happens to the salt crystals.
The salt dissolved in the water. Transparency has not changed. The color has not changed. But the taste – yes! The solution became salty.
Insert a funnel with a filter into an empty glass and pass water and salt through it. The salt and water passed through the filter; it did not remain on the filter. And the taste after filtering is the same. So she dissolved.
Let's conduct an experiment: Fill a transparent glass with boiled water. Pour a teaspoon of granulated sugar into it. While stirring the water, observe what happens to the sugar crystals.
Sugar dissolved in water. The transparency of the water has not changed. The color has not changed. Sugar was no longer visible in the water. But the taste – yes!
Insert a funnel with a filter into an empty glass and pass water and sugar through it. Sugar dissolved in water. It did not remain on the filter, it passed along with the water. And the taste after filtering is the same.
Let's carry out the experiment: Stir a teaspoon of river sand in a glass of water. Let the mixture sit.
The color of the water changed, it became cloudy and dirty. Large grains of sand lie on the bottom, small ones float. The sand did not dissolve.
Insert a funnel with a filter into an empty glass and pass the contents through it. The sand remained on the filter, the water passed through and was purified. The filter helps purify water from particles that do not dissolve in it.
Let's carry out the experiment: Stir a teaspoon of clay in a glass of water. Let the mixture sit.
The clay has not dissolved in the water, the water is cloudy, large clay particles have fallen to the bottom, and small ones float in the water.
Pass the contents of the glass through a paper filter. Water passes through the filter, and undissolved particles remain on the filter. The filter helped clean the water from particles that did not dissolve in the water.