Medication for type 2 neurofibromatosis. Neurosurgical aspects of type II neurofibromatosis
Type 2 neurofibromatosis (NF2) is much less common than NF1 neurofibromatosis with an incidence of 1: 33000 to 1: 50,000. NF2 is inherited in an autosomal dominant manner, with gene penetrance over 95%. NF2 often manifests itself in the second and third decades of life. According to Kanter et al., The average age at onset of symptoms of vestibular schwannoma is 20.4 years.
On the vestibular schwannomas accounts for about 8% of intracranial tumors and about 80% of angular cerebellopontine tumors. Most vestibular schwannomas are sporadic and unilateral, manifesting in the fifth decade.
Patients with neurofibromatosis type 2(NF2) and bilateral vestibular schwannomas account for 2 to 4% of all cases of vestibular schwannomas.
Type 2 neurofibromatosis very often confused with NF1. Final proof that NF1 and NF2 are distinct entities was not possible until 1987. Many, including molecular biological studies carried out by Barker et al., Have shown that the gene responsible for the development of NF1 is localized near the proximal end of the long arm of chromosome 17. An independent study conducted during the same period by Rouelau et al. showed that the gene responsible for NF2 is localized on chromosome 22.
Type 2 neurofibromatosis develops as a result of inheritance of a gene mutation located on chromosome 22. The frequency of mutations within the gene is estimated to be 6.5 x 10 -6. In these patients, in about 50% of cases, there is no family history and new generative mutations develop. It was chromosome 22 that came to the fore as a likely source of NF2 after cytogenetic studies of meningiomas in 1982. The HF2 gene was later assigned to chromosome 22 in both linkage and heterozygosity analysis, respectively in 1986 and 1987.
IN 1993 year two independent groups of researchers have isolated the NF2 gene, called merlin or schwannomine. The HF2 gene extends over 100 kb on chromosome 22q12.2 and contains 17 exons. The messenger RNA coding sequence is 1785 bp in length and encodes a 595 amino acid protein. The gene product is close to the family of proteins, which include moesin, ezrin, radixin, talin, and others that are part of the 4.1 protein subfamily. These proteins are involved in the adhesion of cytoskeleton components to the cytoplasmic membrane and are localized in actin-rich microvilli. It is believed that the N-terminal region of the merlin protein interacts with the components of the cytoplasmic membrane, and the C-terminal region interacts with the cytoskeleton.
Merlin or Schwannomine is predominantly expressed cells of the nervous system and in the lens... It is currently believed that overexpression of the protein merlin can inhibit cell growth and this contributes to the formation of protrusions on the cell surface and cell elongation. In 1994, Tikoo et al. tested the ability of merlin as a tumor gene suppressor. Subsequently, by presenting the NF2 protein in v-Ha-Ras-transformed NIH 3T3 cells, they were able to demonstrate the elimination of the malignant phenotype, and thus confirm the ability of merlin to suppress tumor.
Although accurate NF2 protein functions not fully understood, the available data indicate that it is involved in intercellular or cell-membrane interactions and plays an important role in the movement, shape and interaction of the cell. As a result of the loss of functions of the protein merlin, a violation of contact inhibition can be observed, which leads to oncogenesis.
Mutations involving gene NF2 are observed in 22-59% of patients with sporadic vestibular schwannoma. Welling et al. compared the frequency of detecting a genetic mutation in patients with sporadic vestibular schwannoma and in patients with NF2 and found a significant difference of 66 and 33%, respectively, noting that different mutational mechanisms can occur in oncogenesis. To date, more than 200 mutations of the NF2 gene have been identified, including single base substitutions, insertions and deletions. Most mutations result in the truncation of the C-terminal end of the protein; of the missense mutations, only 13 have been identified.
Defects gene NF2 found in other neoplasms, including meningiomas, malignant mesotheliomas, melanomas, and breast cancer.
Study genotype-phenotype correlations suggests that mutations in the NF2 gene are associated with different phenotypic expression. Ruttledge et al. found that mutations in the HF2 gene resulting from protein clipping are associated with more severe clinical manifestations of HF2 (Wishart), while missense and splice site mutations are associated with milder disease progression (Gardner).
Parry et al. also indicated that retinal lesions are associated with more destructive gene protein clipping mutations. Both studies found intrafamilial differences in phenotypic expression.
Though NF2 gene mutations play a leading role in the nature of vestibular schwannomas, it is possible that other genetic loci contribute to the development of vestibular schwannomas. In addition to the above, it should be noted that mutations in the gene for neurofibromatosis type 2 (NF2) are found in all patients with vestibular schwannoma, while genotypic-phenotypic correlations are possible, the existence of which suggests that other genetic loci may also contribute to the occurrence of vestibular schwannomas and the final phenotype of the patients.
Gene detection, responsible for NF2 largely depends on our understanding of the pathology at the molecular level and the factors responsible for clinical heterogeneity among patients with neurofibromatosis type 2 (NF2). Soon, understanding the functions of merlin in tumor formation will become the basis for the development of antitumor therapy, which can ultimately alleviate the suffering of patients associated with neurofibromatosis type 2 (NF2).
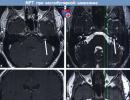
 MPT with contrast enhancement, T1 mode.
MPT with contrast enhancement, T1 mode. Intracanalicular vestibular schwannoma on the left (VS, arrow) in (a) axial and (c) frontal sections.
Vestibular schwannoma (VS) is located in the area where there is no signal from the cerebrospinal fluid (arrow) in the area of the internal auditory canal on (b) high-resolution T2-weighted tomograms in the CISS sequence.
In T1 mode without contrast, the VS is not visualized. (d) The arrow indicates the location of the tumor, which is not visible.
Type II (second) neurofibromatosis (NF2)- an autosomal dominant hereditary disease that is inherited or occurs spontaneously, characterized by the formation of multiple benign tumors, mainly schwannomas and meningiomas, localized in the central nervous system and along the peripheral nerves. Despite the benign nature of the neoplasms, the disease is often fatal due to the formation of inoperable intracranial tumors that impair brain function. There is no medical treatment for this disease, moreover, patients are forced to undergo multiple surgical interventions to remove tumors, which sooner or later leads to deafness and blindness. The development of the disease is associated with gene damage NF2... Mutations in this gene are associated with both the formation of sporadic schwannomas and meningiomas throughout the body, and the development of tumors that, at first glance, are not associated with neurofibromatosis, for example, malignant mesotheliomas.
Epidemiology
Type II neurofibromatosis occurs in 1 in 50 thousand newborns.
Etiology
The NF2 gene is localized on the long arm of chromosome 22 (22q12) and encodes the synthesis of a tumor growth suppressor - the protein merlin or schwannomine. The properties and structure of merlin are very close to three homologous proteins - ezrin, radixin and moesin (from where it got its name M oezin E zrin R adixin L ike prote IN ). All of these proteins function as membrane organizers and primarily provide for the construction and functioning of the cell skeleton (microtubule system). These proteins are of the greatest importance in the regulation of cell proliferation of neuroectodermal origin.
The mutation of one NF2 gene encoding the synthesis of merlin does not manifest itself at the cellular level, since the allelic gene produces RNA for the synthesis of a protein sufficient for the needs of the cell. When it is damaged (as a result of the second genetic event), the synthesis of normal merlin in the cell stops, the dynamic balance of growth regulation shifts towards proliferation and benign tumor growth occurs.
Clinical picture
Tumors arising in type II neurofibromatosis are benign, but more biologically aggressive compared to neoplasms in type I neurofibromatosis. The likelihood of developing associated malignant tumors in patients with NF2 increases insignificantly.
Diagnostics
The absolute diagnostic criterion for NF2 is bilateral neurinomas of the VIII nerve. Also, the diagnosis of NF2 is established when determining in a patient who has a direct relative with this disease, either a unilateral neuroma of the VIII nerve, or a combination of two or more of the following signs:
- neurofibromas
- meningiomas (one or more)
- gliomas (one or more)
- schwannomas, including spinal (one or more)
- juvenile posterior subcapsular lenticular cataract or lens opacity
Café-au-lait spots are observed in about 80% of patients with NF2, but have no diagnostic value.
Treatment
With bilateral neuromas and preserved hearing, it is recommended to start treatment with a tumor of a smaller size, in case of hearing loss - from the side of the better hearing ear. If, after complete removal of the tumor, hearing on this side remains satisfactory, then another tumor should be removed. If hearing could not be preserved, expectant tactics are recommended for the remaining neuroma, and if symptoms increase, partial removal of the tumor (due to the high risk of deafness).
If neuromas and neurofibromas not associated with NF2 only displace the auditory nerve, then with NF2 a tumor in the form of grape bunches often spreads between the fibers of the 8th nerve, which makes it difficult to preserve hearing in these patients. Also, in NF2, it is difficult to separate the tumor from other cranial nerves, primarily from the facial one.
In the presence of other intracranial neoplasms, their surgical removal is indicated, if the localization and size of the formation allow it, or radiosurgical treatment.
85.0Type II (second) neurofibromatosis (NF2)- a hereditary disease that predisposes to the development of tumors in humans.
Epidemiology
Type II neurofibromatosis occurs in 1 in 50 thousand newborns.
Etiology
The NF2 gene is localized on the long arm of chromosome 22 (22q12) and encodes the synthesis of a tumor growth suppressor - the protein merlin or schwannomine. The properties and structure of merlin are very close to three homologous proteins - ezrin, radixin and moesin (from where it got its name M oezin E zrin R adixin L ike prote IN ). All these proteins function as membrane organizers and primarily provide for the construction and functioning of the cell skeleton (microtubule system). These proteins are of the greatest importance in the regulation of cell proliferation of neuroectodermal origin.
Mutation of one NF2 gene encoding merlin synthesis does not manifest itself at the cellular level, since the allelic gene produces RNA for the synthesis of a protein sufficient for the needs of the cell. When it is damaged (as a result of the second genetic event), the synthesis of normal merlin in the cell stops, the dynamic balance of growth regulation shifts towards proliferation and benign tumor growth occurs.
Clinical picture
Tumors arising in type II neurofibromatosis are benign, but more biologically aggressive compared to neoplasms in type I neurofibromatosis. The likelihood of developing associated malignant tumors in patients with NF2 increases insignificantly.
Diagnostics
The absolute diagnostic criterion for NF2 is bilateral neuromas of the VIII nerve. Also, the diagnosis of NF2 is established when determining in a patient who has a direct relative with this disease, either a unilateral neuroma of the VIII nerve, or a combination of two or more of the following signs:
- meningiomas (one or more)
- gliomas (one or more)
- schwannomas, including spinal (one or more)
- juvenile posterior subcapsular lenticular cataract or lens opacity
Café-au-lait spots are observed in about 80% of patients with NF2, but have no diagnostic value.
Treatment
With bilateral neuromas and preserved hearing, it is recommended to start treatment with a tumor of a smaller size, in case of hearing loss - from the side of the better hearing ear. If, after complete removal of the tumor, hearing on this side remains satisfactory, then another tumor should be removed. If hearing could not be preserved, expectant tactics are recommended for the remaining neuroma, and if symptoms increase, partial removal of the tumor (due to the high risk of deafness).
If neuromas and neurofibromas not associated with NF2 only displace the auditory nerve, then with NF2 a tumor in the form of grape bunches often spreads between the fibers of the 8th nerve, which makes it difficult to preserve hearing in these patients. Also, in NF2, it is difficult to separate the tumor from other cranial nerves, primarily from the facial one.
In the presence of other intracranial neoplasms, their surgical removal is indicated, if the localization and size of the formation allow it, or radiosurgical treatment.
Neurofibromatosis is a genetic disorder characterized by abnormal growth of feeder cells (Schwann cells), with the formation of smaller and larger tumors.
Tumors can be benign or malignant. Neurofibromatosis is a relatively rare disease, affecting 1 in 2,500-4,000 babies born. It is an autosomal dominant hereditary disorder. The disease usually occurs in 2 main forms (types 1 and 2, older names - peripheral and central).
In most cases, a congenital mutation plays a role as the cause of the development of the disease, but it is also possible that the disease develops as a result of the emergence of new mutations. The diagnosis can be accurately determined using genetic analysis.
Finding the origins of the problem
Neurofibromatosis is an inherited autosomal dominant disorder. This means that with a certain combination of genes, the disease is inherited from the parents. In the case of autosomal dominant inheritance, both sexes are affected by the disease equally often.
The disease is transmitted by genes located on non-sex chromosomes - autosomes (chromosome 17 is most typical for type 1 neurofibromatosis, 22 for type 2).
Another, less common, option is the emergence of new mutations. This means that the disease first appears in humans with the formation of new mutations. His parents or other relatives do not suffer from this disease, but the person himself subsequently passes the disease on to his descendants.
Varieties of the disease
Neurofibromatosis is divided into 2 main types:
- Type NF1 also known as von Recklinghausen disease. The whole disease occurs in a ratio of 1: 3000 people.
- Type NF2 is a rarer phenomenon, affects 1 person out of 25,000.
Also, 4 more types of the disease are distinguished, but they are extremely rare and the scheme of their treatment does not differ from the treatment of the second type of disease.
Both types form separate diseases that have different causes and symptoms.

Symptoms and manifestations
Both types of the disease manifest themselves in different ways and the nature of the clinical picture is different.
Von Recklinghausen's disease
Recklinghausen neurofibromatosis manifests itself in children and has the following symptoms:
- White coffee character skin patches... Light brown spots on the skin are painless. Spots in childhood grow up to 5 mm, in adolescence - they increase in size up to 15 mm.
- Freckles with this type, they occur in unusual places, for example, in the folds of the skin.
- Benign skin tumors - neurofibromas... These are benign tumors that grow under the skin. In childhood, they are small, with increasing age, as a rule, they become larger. The number of neurofibromas varies from person to person; in some, these tumors cover the entire body. Some of them cause persistent itching, shape changes or dysfunction of the limbs.
- Optic nerve glioma... Glioma is a benign tumor of the optic nerve that causes visual impairment and in children it causes a change in color perception.
- Lisha's nodules... They are brown spots on the iris of the eye.
- High blood pressure.
- Malignant neoplasms of the peripheral nerve sheath... Each nerve has its own sheath. A suspicion of malignancy is present if the neurofibroma suddenly swells, becomes painful, weakness, mood changes, and tingling in the extremities appear.

Type 2 neurofibromatosis
The first manifestations of this type of disorder usually occur after the age of 20; in young children, the disease usually does not cause any symptoms.
Among the characteristic symptoms, the following violations are noted: 
- hearing loss, buzzing and tingling in the ears;
- problems maintaining balance;
- dizziness and vomiting are often present;
- the growth of tumors in the ear area that damage hearing and the balance of the nerves that transmit signals to the brain.
In some people, it grows directly in the brain, but does not always manifest; we are talking about benign neoplasms. The problem arises when the tumor grows to a large size and oppresses the surrounding brain tissue. This can manifest itself as headache, dizziness, vomiting.
They can also develop, which can cause:
- back pain;
- muscle weakness;
- tingling in the limbs, numbness.
Benign tumors in NF2 look like a raised skin covering about 2 cm in diameter.
Manifestations of rare types of the disease
Symptoms of other types of neurofibromatosis:
- disease 3 types characterized by the appearance of a number of skin neurofibromas, which can lead to optic nerve glioma, neurolemma and;
- 4 type the disease is segmental, and affects only one specific area of the skin;
- neurofibromatosis 5 types characterized by the absence of neurofibromas and is manifested by the presence of only dark spots;
- 6 type the disease is characterized by the appearance (as in the case of type 2 neurofibromatosis) after 20 years of age. Neurophims appear; the disease is most often acquired.

Diagnostic criteria and genetic analysis
When diagnosing neurofibromatosis, it is important to know that we are talking about a hereditary disease with various manifestations. Based on this information, the so-called. diagnostic criteria aimed at helping in the "disclosure" of this disease.
The first in the list of criteria are cafe-au-lait spots (“white coffee”), for which the number of 6 pieces or more is indicated in the size of 5 millimeters or more. Another characteristic suggests the presence of 2 or more neurofibromas, multiple freckles in the folds of the skin (in the armpits and groin), and optic nerve gliomas.
The presence of two or more Leesch spots and bone dysplasia also play an important role in the diagnosis. And, finally, the frequency of the disease in the family is no less important, especially in relation to parents, brothers and sisters. The incidence of the above characteristics increases with the age of the patient.
 Most often, it is possible to diagnose neurofibromatosis in childhood, up to 4 years of age. Usually the diagnosis is based on a typical clinical picture, but in case of doubt or uncertainty, you can resort to the help of genetics. In this case, DNA or RNA analysis can be used to detect the disease.
Most often, it is possible to diagnose neurofibromatosis in childhood, up to 4 years of age. Usually the diagnosis is based on a typical clinical picture, but in case of doubt or uncertainty, you can resort to the help of genetics. In this case, DNA or RNA analysis can be used to detect the disease.
Collection of peripheral venous blood is sufficient for this examination. When the disease occurs in one of the parents-to-be, prenatal genetic testing is possible, which is often done with amniocentesis. In addition, you can pre-implantation study the eggs or sperm, immediately before fertilization and conception of the child.
Childhood disease
There are 3 different types of neurofibromatosis that children can develop. Each of them develops due to a genetic defect present in the genes or arising immediately after conception.
The type 1 neurofibromatosis gene is present on chromosome 17 and increases the production of the protein neurofibromin. This protein helps control cell growth in the nervous system. Mutation of the NF1 gene results in protein loss and cells grow abnormally.
The HF2 gene is present on chromosome 22 and affects the production of the Merlin protein. The NF2 mutation leads to a loss of protein, as a result of which it comes to uncontrolled cell growth in the nervous system.
The SMARCB1 gene is present on chromosome 22 and is the cause of schwannomatosis.
Features of the clinic for pediatric disorders
Each type of neurofibromatosis has different signs and symptoms.
The first type of the disease is most often manifested in a child. Visible symptoms of type 1 neurofibromatosis in children include:

Neurofibromatosis 2 (NF2) mainly affects a child's ears:
- gradual hearing loss;
- tinnitus;
- imbalance.
In some rare cases, NF2 can also affect the spinal cord and peripheral nerves. The symptoms in this case are as follows:
- strong pain;
- numbness or weakness in the arms or legs.
Schwannomatosis is a rare form of neurofibromatosis that rarely occurs in young children. This variant of the disease usually develops at an older age and causes neoplasms on the spine, cranial or peripheral nerves.
In the presence of this form of the disease, chronic pain in any part of the body can occur.
How can you help a person?
Neurofibromatosis is a genetic disease and, unfortunately, incurable. Tumor formation occurs on the basis of congenital changes in DNA, and today there is no way to influence this process in any way.
Drug treatment involves taking the following drugs:
- Ketotifen;
- Fenkarol;
- Tigazon to reduce the rate of cell division;
 In the event of a tumor that bothers the patient (there is pressure in the area of the neoplasm due to ingrowth into healthy tissue, closure of the gastrointestinal tract occurs, or the tumor is cosmetically unpleasant for a person), it can be surgically removed. Usually the surgeon tries to remove the entire tumor. However, there is no guarantee that it will not appear elsewhere.
In the event of a tumor that bothers the patient (there is pressure in the area of the neoplasm due to ingrowth into healthy tissue, closure of the gastrointestinal tract occurs, or the tumor is cosmetically unpleasant for a person), it can be surgically removed. Usually the surgeon tries to remove the entire tumor. However, there is no guarantee that it will not appear elsewhere.
Tumors in the brain are problematic, which can depress important areas of the brain and cause devastating complications of vision, hearing, the motor system, and trigger paralysis or headaches.
In the head area, in addition to open surgical intervention by opening the skull and removing the tumor, you can also choose the option of a gamma knife that acts on the neoplasm with radiation. If benign tumors develop into malignant metastases, chemotherapy, radiation therapy, and other methods used in oncology may be recommended.
Radiation therapy is usually ruled out due to the occurrence of secondary malignancy (the creation of a new tumor after radiation). The goal of treatment is early detection of the disease with medical examination of the patient, regular check-ups and, if necessary, prompt treatment.
Chemotherapy is usually the first choice after surgical removal. Bone deformity can be adjusted with surgical strengthening or cosmetic correction.
All of the above treatments only serve to improve the quality of life, relieve pain or mental distress, but do not completely cure the disease.
For the treatment of neurofibromatosis with folk remedies, healers recommend taking propolis tincture (100 g of propolis per 500 ml of alcohol). It is infused for a week in a dark place, after which it should be filtered. Take 30 drops daily 3 times. Store the tincture in a dark place at room temperature.
Why is the disease dangerous?
Living with neurofibromatosis, in particular the first type, is very stressful and troublesome, since this disease has  destructive effect on appearance. The disease has a negative psychological effect, even if it is only a slight distortion of the appearance of the skin.
destructive effect on appearance. The disease has a negative psychological effect, even if it is only a slight distortion of the appearance of the skin.
This is especially true for adolescents who are very sensitive to their appearance, the manifestations of the disease cause a feeling of shame and lead to depression and anxiety.
In addition, complications can be more serious: tumor tissue begins to grow very quickly and its cells spread to other parts of the body (metastasis).
How not to face a dangerous disease?
Preventive measures for neurofibromatosis are a complex issue that includes several options. In the case of a congenital disorder, there is no known one hundred percent way to prevent its development.
If there is a family history of neurofibromatosis, then for a couple planning a child, there is the possibility of genetic analysis. It is necessary to create a family tree and mark all already sick persons.
It is also good to know what type of disease we are talking about in the framework of prenatal genetic diagnostics of the examination of the unborn child. Preimplantation genetic testing is able to examine the embryo prior to implantation into the uterus.
If we are talking about a disease acquired during a lifetime, it is theoretically possible to avoid everything that can affect human genetics (radiation, chemical and toxic substances, etc.), but, unfortunately, without a guarantee of success.
The above possibilities of preventing the disease may seem like something supernatural, but, despite this, they are somewhat limited, and can not always prevent the development of the disease by 100%.
(NF) is a hereditary disease that predisposes to the development of tumors in humans.
In the literature, neurofibromatosis was first described in 1822 by the Scottish surgeon Wishart, who described a patient with type 2 NF. Type 1 neurofibromatosis was studied and described in 1882 by Virchow's student von Recklinghausen. However, in 1916, Cushing in his scientific work combined these diseases under the general name "Recklinghausen's disease", and only after molecular genetic studies, the results of which were published in 1985 and 1987, were fundamental differences in the pathogenesis of NF1 and NF2 revealed. It has been proven that these are completely different diseases that require a differentiated clinical approach.
The literature describes only eight "types" of neurofibromatosis, but recently most of them (except for NF2) are considered abortive forms of NF1 and are not distinguished as independent nosological forms. Exceptions may be segmental neurofibromatosis (NF5), when typical manifestations of NF1 are localized in one or more neighboring dermatomes (extremely rare, usually not inherited), and, not included in the number of eight, spinal neurofibromatosis, in which all spinal roots are symmetrically affected (described only a few observations).
Neurofibromatosis type 1 (NF1) is an autosomal dominant disorder (incidence in the population is 1 in 3500 newborns). In all cases of NF1, the genetic defect is localized in zone 11.2 of chromosome 17 (17q11.2). The NF1 gene located here encodes the synthesis of a large protein - neurofibromin, which is involved in the inactivation of promoter proteins (ras-protein and its analogs), providing dynamic control of cell growth. The NF1 gene is one of the main tumor suppressor genes for about 30% of body tissues, primarily of neuroectodermal origin. When the NF1 gene is damaged in one of the chromosomes of the 17th pair, 50% of the synthesized neurofibromin becomes defective, and a shift in the balance of cell growth towards proliferation is observed.
We would like to focus on type 2 neurofibromatosis, which was previously called "central neurofibromatosis" and which predisposes to the appearance of benign neoplasms in the central nervous system.
Neurofibromatosis type 2 (NF2), like NF1, is an autosomal dominant disease, but it occurs much less frequently in the population (1 case per 40,000 newborns). The genetic defect in this disease is fundamentally in a different chromosome and, therefore, the pathogenesis of this disease is different.
The HF2 gene is localized on chromosome 22 (22q12) and encodes the synthesis of another tumor growth suppressor, the protein merlin, which functions as a membrane organizer and primarily ensures the construction and functioning of the cell skeleton. This protein is of greatest importance in the regulation of cell proliferation of neuroectodermal origin.
Mutation of the NF2 gene encoding merlin synthesis in one chromosome does not manifest itself at the cellular level, because a 50% decrease in merlin synthesis is leveled by ERM proteins, which are also involved in the regulation of cell proliferation. However, when the HF2 allelic gene is damaged (as a result of a "second genetic event" - symmetric mutation or loss of heterozygosity on chromosome 22), the synthesis of normal merlin in the cell stops, the dynamic balance of growth regulation shifts towards proliferation and benign tumor growth occurs.
Given the presence of many nonspecific symptoms in patients, in 1987 for the diagnosis of "NF2", the US National Institute of Health developed absolute diagnostic criteria (NIH criteria), and later added probable criteria to them (Table 1).
Table 1. Diagnostic criteria for neurofibromatosis type 2 (includes NIH criteria and probable criteria).
Absolute signs | Likely signs |
||
Bilateral vestibular schwannomas (neurinoma of the VIII cranial nerve) | Family history | Unilateral vestibular schwannoma Age less than 30 years Any two of these (meningioma, glioma, neurofibroma, schwannoma, posterior subcapsular lenticular cataract) | Multiple meningiomas (two or more) Unilateral vestibular schwannoma Two or more of these tumors (glioma, neurofibroma, schwannoma) or Cataract |
According to Antinheimo et al, 3% of patients with schwannomas and 1% of patients with meningiomas have neurofibromatosis 2. 20% of patients with multiple meningiomas have NF2 [2].
Clinical manifestation of the disease.
The most common manifestation of type 2 neurofibromatosis is the presence of bilateral vestibular schwannomas. The second most common tumors are schwannomas of other cranial, spinal and peripheral nerves. Much less frequently (less than 10%) are meningiomas (intracranial, including meningiomas of the optic nerves, and spinal), epindymomas and gliomas.
In principle, schwannomas can form anywhere in the body where there are nerves with Schwann cells. The preferred localization of tumors on the VIII nerve in NF2 remains unexplained to this day.
Most often, patients go to the doctor in connection with hearing loss or with the appearance of tinnitus, which are unilateral at the onset of the disease. These complaints may be accompanied by dizziness and ataxia. In 20-30% of cases in these patients, in addition to vestibular schwannomas, meningiomas, spinal or peripheral tumors are detected.
Often, the disease manifests itself with Bell's palsy (3-5%), which does not respond to treatment and several years pass before the cause of its appearance is identified. Some patients develop a poliomyelitis-like syndrome (about 3%).
60-80% of patients with type 2 neurofibromatosis have visual impairments - cataracts, retinoblastomas, hemarthromas, meningiomas of the optic nerves, etc.
Approximately 70% of patients have changes in the skin and distal branches of the peripheral nerves (coffee-and-milk spots, schwannomas, neurofibromas)
Meningiomas with neurofibromatosis type 2 are more often localized supratentorially and are located mainly on the falciform process in the frontal and parietal regions. No regularity in the occurrence of a particular type of meningioma has been identified. The presence of spinal meningiomas is also characteristic. No correlation was found between the histological type of meningioma and the presence of NF2.
The frequent combination of NF2 and meningiomas is explained by the presence of a genetic defect on one chromosome. In sporadic meningiomas, mutations in the NF2 gene on chromosome 22 occur in 30-60%.
Cases of mixed tumors consisting of meningioma and schwannoma cells have been described. As a rule, these tumors are localized in the area of the cerebellar pontine angle. Also, with NF2, meningoangiomatosis is often detected.
In 8% of cases, meningiomas are the first neoplasms before the appearance of a neuroma of the VIII cranial nerve. Genetic examination of patients with meningiomas often (in 90% of cases) reveals a mutation in chromosome 22.
Low-grade epindymomas and gliomas in NF2 are much less common, and tumors are localized mainly in the brainstem and in the upper cervical segments of the spinal cord. Malignancy of these tumors is rare and, in most cases, is associated with radiation therapy.
Surgical tactics.
With bilateral neuromas and preserved hearing, it is recommended to start treatment with a tumor of a smaller size, in case of hearing loss - from the side of the better hearing ear. If, after complete removal of the tumor, hearing on this side remains satisfactory, then another tumor should be removed. If hearing could not be preserved, expectant tactics are recommended for the remaining neuroma, and if symptoms increase, partial removal of the tumor (due to the high risk of deafness).
If neuromas and neurofibromas not associated with NF2 only displace the auditory nerve, then with NF2 a tumor in the form of grape bunches often spreads between the fibers of the 8th nerve, which makes it difficult to preserve hearing in these patients. Also, in NF2, it is difficult to separate the tumor from other cranial nerves, primarily from the facial one.
In the presence of other intracranial neoplasms, their surgical removal is indicated, if the localization and size of the formation allow it, or radiosurgical treatment.
CASE FROM PRACTICE
Here is a description of the successful treatment of a patient with NF 2 and multiple intracranial and extracranial masses.
Patient L., 27 years old, hospitalized in the neurosurgical department of the State Institution of the Republican Scientific Center of Chemistry of the RAMS named after BV Petrovsky 03/29/2006 complaining of a headache that worsens in the morning and when bending forward, exophthalmos on the right, pain in the right eye, decreased vision, double vision, recurrent "congestion" of the right half of the nose.
From the anamnesis it is known that about 6 months ago the patient discovered a small exophthalmos on the right, for which she was examined and treated by ophthalmologists at the place of residence, but the exophthalmos increased. After 2-3 months, a headache appeared, localized in the frontal region and the orbit area, which was not relieved by the intake of NSAIDs. Gradually the headache increased, periodically, for no reason, difficulty in nasal breathing on the right began to appear. On an outpatient basis, CT of the brain was performed, which revealed a mass in the basal parts of the frontal region, spreading through the ethmoid labyrinth into the nasal cavity, and growing through the medial wall into the cavity of the right orbit. The family history is not burdened, among the patient's relatives no oncological diseases have been identified so far.
The general condition of the patient upon admission is satisfactory. No pathology was revealed in the patient's somatic status.
Neurological status: consciousness is clear, oriented, adequate. GCS - 15 points. There are no meningeal symptoms. Right-sided exophthalmos, pupils OS = OD, photoreaction and corneal reflexes are vivid. Restriction of upward and medial movement of the right eyeball, impaired convergence on the right, diplopia. Visus OD = 0.7, OS = 1. The face is symmetrical, the tongue is in the midline. Tendon reflexes from the extremities are somewhat revitalized on the left, muscle tone without difference in sides. There are no paresis or sensory disturbances. Performs coordination tests with intention.
When examining the patient, attention is drawn to the right-sided exophthalmos, the movements of the left eyeball are painful, on the right they are somewhat limited upward and medially. On the lateral wall of the nose, at the top, near the right eye socket, under the skin, the formation of a dense consistency with even smooth edges is palpable. On the palmar surface of the right hand there is a subcutaneous formation of a rounded shape, measuring 0.7 x 0.5 x 0.5 cm, not displaced with the skin and moderately painful on palpation. The same subcutaneous formations were found on the middle phalanx of the second finger of the left hand and in the occipital region.
No pathology was found on the fundus.
The patient was examined by an otolaryngologist - on the lateral wall of the right nasal passage, in the posterior-upper sections, a dense submucosal formation was found 4-5 mm protruding into the cavity of the nasal passage.
The patient underwent an MRI of the brain with contrast, which revealed:
In the projection of the ethmoid bone, an inhomogeneous zone of the altered MR signal is revealed (hypointense on T1-weighted images and hyperintense on T2-weighted images) with a total size of 77 x 35 x 39 mm, irregular shape with spreading into the right orbit (with compression and displacement of the inner and upper oblique eye muscles outward and outward displacement of the eyeball) and the upper nasal passage on the right (with destruction of bone structures), into the frontal sinuses and soft tissues of the right half of the nose. Expanding into the cranial cavity extradurally, the formation compresses and displaces the frontal lobes posteriorly and laterally. The MR signal from the intracranial part of the lesion is sharply increased in T1 and T2-weighted images.
In the frontal parts of the interhemispheric sulcus, a rounded structure with clear even contours is determined, emanating from the crescent of the brain, isointense on T1 and T2 images. The size of the formation is about 8 mm.
At the level of the craniovertebral junction, inside the spinal canal to the left and behind of the medulla oblongata, intimately adjacent to it, a round-shaped volumetric mass with clear even contours is determined, isointensive on T1 and T2-weighted images with dimensions of 6 x 10 mm.
After the introduction of a contrast agent, its intense heterogeneous accumulation of education in the region of the nose and paranasal sinuses is noted. Other formations accumulate contrast evenly. The MRI data were regarded by specialists as polyposis (?) Complicated by secondary mycotic infection and hemorrhage.
After an additional examination, on April 6, 2006, the patient underwent surgery - osteoplastic trepanation in the frontal region, removal of a small convexital meningioma in the right frontal region (detected during the operation), removal of the meningioma of the anterior third of the falx, removal of the craniofacial mass formation.
Under general anesthesia, a lumbar drainage is installed in the L 3-L 4 interval, and a transparent, colorless cerebrospinal fluid flows through the drain. An arcuate incision was made in the soft tissues in the frontal region. Osteoplastic trepanation was performed in the frontal region with additional resection of the frontal bone and the walls of the frontal sinus to the bottom of the anterior cranial fossa. The dura mater is tense, at the bottom of the anterior cranial fossa, a volumetric lesion was found lying extradurally. In the right frontal region at the top of the dura mater, an arc was opened and a small convexital meningioma was found, which was removed with resection of the dura mater in the place of its initial growth. An interhemispheric approach was used to approach the meningioma of the anterior third of the falx, which was located at a depth of 1.5 cm. The meningioma was removed with resection of the falx in the place of its growth. The dura mater defect was plasticized with aponeurosis.
The tumor at the bottom of the anterior cranial fossa was isolated along the periphery; the place of initial tumor growth was the dura mater of the olfactory fossa floor. In this place, the dura mater was excised, after which the intracranial part of the tumor was removed, which was a solid component and a cyst with dense walls. The cavity of the frontal sinuses was filled with a solid component of the tumor, which was removed, the mucous membrane from the frontal sinuses was also removed. Further, the resection of the horizontal plate of the ethmoid bone was performed, the resection of the ethmoid labyrinth, germinated by the tumor. The intranasal part of the tumor was removed by lumping from the cranial cavity, and after removal of the tumor, a direct passage was formed from the cranial cavity to the nasal cavity at the level of the superior concha. The intraorbital part of the tumor was also removed from this approach. Hemostasis at blood pressure 150/90 mm Hg
The defect of the dura mater was plasticized with two layers of aponeurosis with gluing along the periphery of the flap with fibrin glue Tissukol - Kit. By the end of the operation, the brain sinks, pulsates well. The walls of the formed cavity are lined with hemostatic gauze "Surgecel". The bone flap was fixed with titanium plates, the primary plastic surgery of the skull bone defect was performed with a titanium implant. Layer-by-layer wound closure. Iodine, alcohol, ac. bandage. Anterior tamponade of the nose was performed.
Histological examination of the removed neoplasms revealed cells of meningotheliomatous meningioma.
In the postoperative period, the patient underwent conservative therapy in the department, including vascular, antibacterial and anti-inflammatory drugs. For 12 days, the patient had a lumbar drain, which was then removed. The postoperative wound healed primarily. There is no leakage of cerebrospinal fluid from the nose, there is no cosmetic defect on the face.
On 05/02/2006, the patient underwent the second stage of surgical treatment - resection of the occipital bone scales, resection of the posterior half-arch of the atlas, removal of the volumetric formation of craniovertebral localization, primary plasty of the skull bone defect with a titanium implant. Removal of schwannomas on the palmar surface of the right hand and soft tissues of the occipital region.
Under general anesthesia, a linear incision of the soft tissues was made in the cervico-occipital region with an approach to the left side. The scales of the occipital bone, the arch of the atlas, the spinous process and the arch of the second cervical vertebra were skeletonized. The scales of the occipital bone (more on the left) and the posterior half-arch of the atlas were resected. The cerebral duct was opened linearly, the large occipital cistern was opened, the cerebrospinal fluid was released, after which the brain was fused. After opening the dura mater at the level of the caudal part of the trunk on the left and the upper segments of the spinal cord, a round-shaped volumetric mass of gray color, dense consistency, well delimited from the adjacent brain, squeezing and displacing it was found. Small arteries extending from the brain stem tightly adjoined the medial parts of the tumor. Under the microscope, the arteries are bluntly separated from the tumor, partially coagulated. The tumor grew from two nerve roots of the accessory nerve. The nerve roots are coagulated and cut off from the tumor, after which the tumor is removed. The size of the removed tumor is 2 x 1 x 1 cm. Hemostasis at BP 110/70 mm Hg. The dura mater was sutured tightly with interrupted sutures. Layer-by-layer wound closure.
The histological conclusion is schwannoma.
The removal of peripheral masses, which also turned out to be schwannomas, was also performed.
On May 16, 2006, the patient was discharged in satisfactory condition under the supervision of specialists at the place of residence without neurological deficits and without cosmetic defects.
Based on the examination of the patient, MRI data, histological examination data, taking into account the absolute and probable signs of neurofibromatosis 2, together with a geneticist, the patient was diagnosed with type 2 neurofibromatosis, craniofacial meningioma with spread itracranially - into the frontal region, extracranially - into the cavity of the right orbit and the nasal cavity ... Meningioma of the anterior third of the falx. Convexital meningioma in the right parietal region. Schwannoma of the left accessory (XI) nerve. Schwannomas of the distal branches of the peripheral nerves (3). Schwannoma of the soft tissues of the occipital region.
Control MRI of the brain 6 months later revealed no masses in the cranial cavity. MRI of the cervical spine revealed no pathology.
BIBLIOGRAPHY.
- Konovalov A. N., Kozlov A. V. Hereditary diseases contributing to the development of tumors of the base of the skull. In the book "Surgery of tumors of the base of the skull", pp. 169-170.
- Antinheimo J, Haapasalo H, Haltia M, Tatagiba M, Thomas S, Brandis A, Sainio M, Carpen O, Samii M, Jaaskelainen J. Proliferation potential and histological features in neurofibromatosis 2-associated and sporadic meningiomas. J Neurosurg 1997; 87: 610-614.
- Antinheimo J, Sankila R, Carpen O, Pukkala E, Sainio M, Jaaskelainen J. Population based analysis of sporadic and NF2-associated meningiomas and schwannomas. J Neurol 2000; 54: 71-76.
- Baser M, Mautner VF, Thakkar SD, Kluwe L. The natural history of neurofibromatosis 2. Am J Hum Genet 1998; 63 (suppl 4): A63.
- Baser ME, Evans DGR, Jackler RK, Sujansky E, Rubenstein A. Malignant peripheral nerve sheath tumors, radiotherapy, and neurofibromatosis 2. Br J Cancer 2000; 82: 998.
- Baser ME, Friedman JM, Evans DGR. Predictors of survival in neurofibromatosis 2. Am J Hum Genet 1999; 65 (suppl 4): A61.
- Baser ME, Mautner VF, Ragge NK, Nechiporuk A, Riccardi VM, Klein J, Sainz J, Pulst SM. Presymptomatic diagnosis of neurofibromatosis 2 using linked genetic markers, neuroimaging, and ocular examinations. Neurology 1996; 47: 1269-1277.
- Black PM. Meningiomas. Neurosurgery. 1993 Apr; 32 (4): 643-57.
- Chakrabarty A, Franks AJ. Meningioangiomatosis: a case report and review of the literature. Br J Neurosurg. 1999 Apr; 13 (2): 167-73.
- Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM. Epidemiology of intracranial meningioma. Neurosurgery. 2005 Dec; 57 (6): 1088-95
- Crowe FW, Schull WJ, Neal JV. A clinical pathological and genetic study of multiple neurofibromatosis. Springfield, Illinois: Charles C Thomas, 1956.
- Elizabeth J, Menon G, Nair S, Radhakrishnan VV. Mixed tumor of schwannoma and meningioma in a patient with neurofibromatosis-2: a case report. Neurol India 2001; 49: 398-400
- Eljamel MS, Foy PM. Multiple meningiomas and their relation to neurofibromatosis. Review of the literature and report of seven cases. Surg Neurol. 1989 Aug; 32 (2): 131-6.
- Evans DGR, Birch JM, Ramsden R. Paediatric presentation of type 2 neurofibromatosis. Arch Dis Child 1998; 81: 496-499.
- Evans DGR, Huson SM, Donnai D, Neary W, Blair V, Newton V, Harris R. A clinical study of type 2 neurofibromatosis. Q J Med 1992; 84: 603-618.
- Evans DGR, Huson SM, Donnai D, Neary W, Blair V, Newton V, Strachan T, Harris R. A genetic study of type 2 neurofibromatosis in the United Kingdom. II. Guidelines for genetic counseling. J Med Genet 1992; 29: 847-852.
- Evans DGR, Huson SM, Donnai D, Neary W, Blair V, Teare D, Newton V, Strachan T, Ramsden R, Harris R. A genetic study of type 2 neurofibromatosis in the United Kingdom. I. Prevalence, mutation rate, fitness, and confirmation of maternal transmission effect on severity. J Med Genet 1992; 29: 841-846.
- Evans DGR, Huson SM, Ponder M, Strachan T, Harding A. Spinal and cutaneous schwannomatosis is a variant form of type 2 neurofibromatosis. J Neurol Neurosurg Psychiatry 1997; 62: 361-366.
- Evans DGR, Ramsden R, Huson SM, Harris R, Lye R, King TT. Type 2 neurofibromatosis: the need for supraregional care? J Laryngol Otol 1993; 107: 401-406.
- Evans DGR, Wallace A, Trueman L, Wu CL, Ramsden RT, Strachan T. Mosaicism in classical neurofibromatosis type 2: a common mechanism for sporadic disease in tumor prone syndromes? Am J Hum Genet 1998; 63: 727-736.
- Evans G. R., C Watson, A King, A J Wallace and M E Baser. Multiple meningiomas: differential involvement of the NF2 gene in children and adults. J. Med. Genet. 2005; 42; 45-48.
- Feiling A, Ward E. A familial form of acoustic tumour. BMJ 1920; 10: 496-497.
- Fucci MJ, Buchman CA, Brackmann DE, Berliner KI. Acoustic tumor growth: implications for treatment choices. Am J Otol 1999; 20: 495-499.
- Gareth R Evansa, M Sainiob, Michael E Baserc. Neurofibromatosis type 2. J Med Genet 2000; 37: 897-904
- Gottfried Oren N., Viskochil David H., Fults Daniel W., Couldwell William T. Molecular, genetic, and cellular pathogenesis of neurofibromas and surgical implications. Neurosurgery 58: 1-16, 2006.
- Gronholm M. Sainio M. Zhao F. Heiska L. Vaheri A. Carpen O. Homotypic and heterotypic interaction of the neurofibromatosis 2 tumor suppressor protein merlin and the ERM protein ezrin. J Cell Sci 1999; 112: 895-904.
- Gutmann DH. Giordano MJ. Fishback AS. Guha A. Loss of merlin expression in sporadic meningiomas, ependymomas and schwannomas. Neurology 1997; 49: 267-270.
- Hartmann C, Sieberns J, Gehlhaar C, Simon M, Paulus W, von Deimling A. NF2 mutations in secretory and other rare variants of meningiomas. Brain Pathol. 2006 Jan; 16 (1): 15-9
- Hesselager G, Holland EC: Using mice to decipher the molecular genetics f brain tumors. Neurosurgery 53: 685–695, 2003.
- Huynh DP, Mautner V, Baser ME, Stavrou D, Pulst SM. Immunohistochemical detection of schwannomin and neurofibromin in vestibular schwannomas, ependymomas and meningiomas. J Neuropathol Exp Neurol 1997; 56: 382-390.
- Kaiser-Kupfer MI, Freidlin V, Datiles MB, Eldridge R. The association of posterior capsular lens opacities with bilateral acoustic neuromas in patients with neurofibromatosis type 2. Arch Ophthalmol 1989; 107: 541-544.
- Kim DG, Paek SH, Chi JG, Chun YK, Han DH. Mixed tumor of schwannoma and meningioma components in a patient with NF-2. Acta Neurochir (Wien). 1997; 139 (11): 1061-4
- Kumar RA, Baser ME, Evans DGR, Wallace A, Mautner VF, Kluwe L, Rouleau G, Joe H, Friedman JM. Intrafamilial correlation of clinical manifestations in neurofibromatosis 2 (NF2). Am J Hum Genet 1999; 65 (suppl 4): A155.
- Martuza RL, Ojemann RG. Bilateral acoustic neuromas: clinical aspects, pathogenesis and treatment. Neurosurgery 1982; 10: 1-12.
- Mautner VF, Lindenau M, Baser ME, Hazim W, Tatagiba M, Haase W, Samii M, Wais R, Pulst SM. Spinal tumors in patients with neurofibromatosis type 2: MR imaging study of frequency, multiplicity, and variety (published erratum appears in AJR 1996; 166: 1231). AJR 1995; 165: 951-955.
- Mautner VF, Lindenau M, Baser ME, Kluwe L, Gottschalk J. Skin abnormalities in neurofibromatosis 2. Arch Dermatol 1997; 133: 1539-1543.
- Mautner VF, Lindenau M, Baser ME, Tatagiba M, Haase W, Samii M, Wais R, Pulst SM. The neuroimaging and clinical spectrum of neurofibromatosis 2. Neurosurgery 1996; 38: 880-885.
- Mautner VF, Tatagiba M, Guthoff R, Samii M, Pulst SM. Neurofibromatosis 2 in the pediatric age group. Neurosurgery 1993; 33: 92-96.
- National Institutes of Health Consensus Development Conference Statement on Neurofibromatosis. Arch Neurol 1987; 45: 575-579.
- Otibi M, Rutka JT. Neurosurgical implications of neurofibromatosis Type I in children. Neurosurg Focus. 2006 Jan 15; 20 (1)
- Parry DM, Eldridge R, Kaiser-Kupfer MI, Bouzas EA, Pikus A, Patronas N. Neurofibromatosis 2 (NF2): clinical characteristics of 63 affected individuals and clinical evidence for heterogeneity. Am J Med Genet 1994; 52: 450-451.
- Ragel BT, Jensen RL. Molecular genetics of meningiomas. Neurosurg Focus. 2005 Nov 15; 19 (5)
- Ragge NK, Baser ME, Klein J, Nechiporuk A, Sainz J, Pulst SM, Riccardi VM. Ocular abnormalities in neurofibromatosis 2. Am J Ophthalmol 1995; 120: 634-641.
- Ruttledge MH, Rouleau GA. Role of the neurofibromatosis type 2 gene in the development of tumors of the nervous system. Neurosurg Focus. 2005Nov; 19 (5).
- Slattery WH, Brackmann DE, Hitselberger W. Hearing preservation in neurofibromatosis type 2. Am J Otol 1998; 19: 638-643.
- Thomas PK, King RHM, Chiang TR, Scaravilli F, Sharma AK, Downie AW. Neurofibromatous neuropathy. Muscle Nerve 1990; 13: 93-101.
- Tomita T, Radkowski MA, Gonzalez-Crussi F, Zaparackas Z, Flannery A. Multiple meningiomas in a child. Surg Neurol. 1988 Feb; 29 (2): 131-6.
- Tsukita S, Yonemura S. Cortical actin organization: lessons from ERM (ezrin / radixin / moesin) proteins. J Biol Chemistry 1999; 274: 34507-34510.
- Turgut M, Palaoglu S, Ozcan OE. The neurosurgical aspects of neurofibromatosis 2: diagnosis and management. Neurosurg Rev. 1998; 21 (1): 23-30.
- Zang KD. Cytological and cytogenetic studies on human meningioma. Cancer Genet Cytogenet 1982; 6: 249-274.
- Zucman-Rossi J, Legoix P, Sarkissian HD, Cheret G, Sor F, Berardi A, Cazes L, Giraud S, Ollagnon E, Lenoir G, Thomas G. NF2 gene in neurofibromatosis type 2 patients. Hum Mol Genet 1998; 7: 2095-2101.